Category Archives: Modern Physics
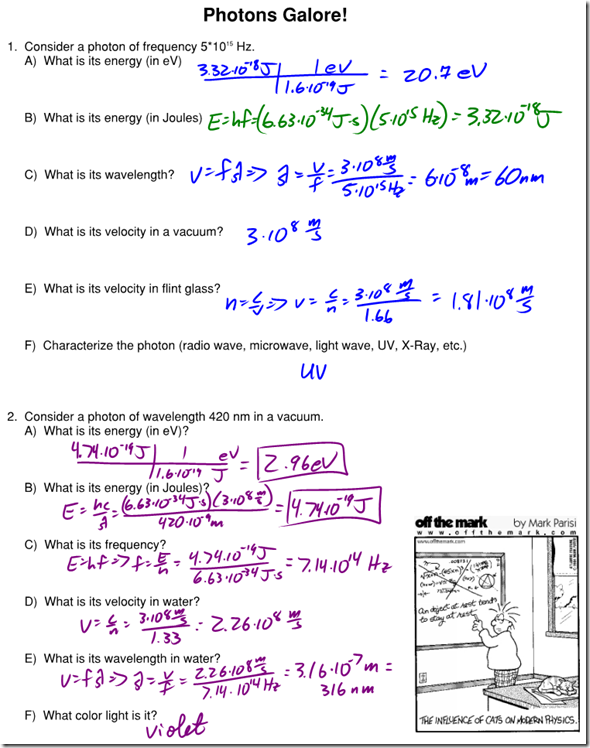
Photons Galore WS
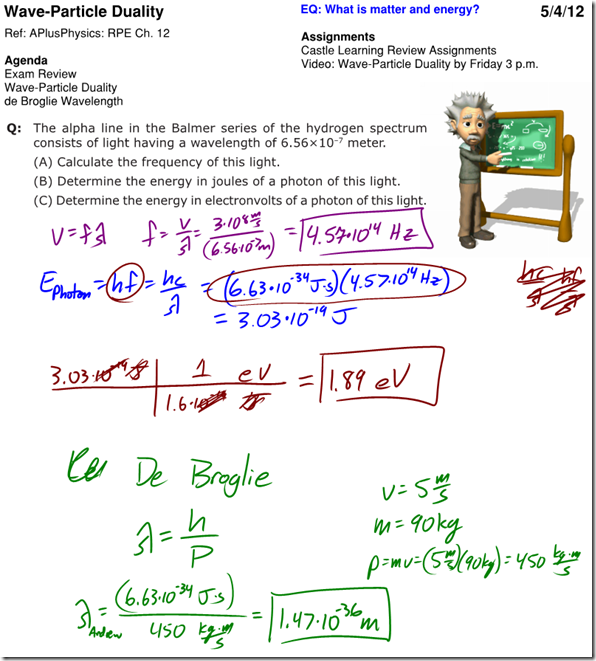
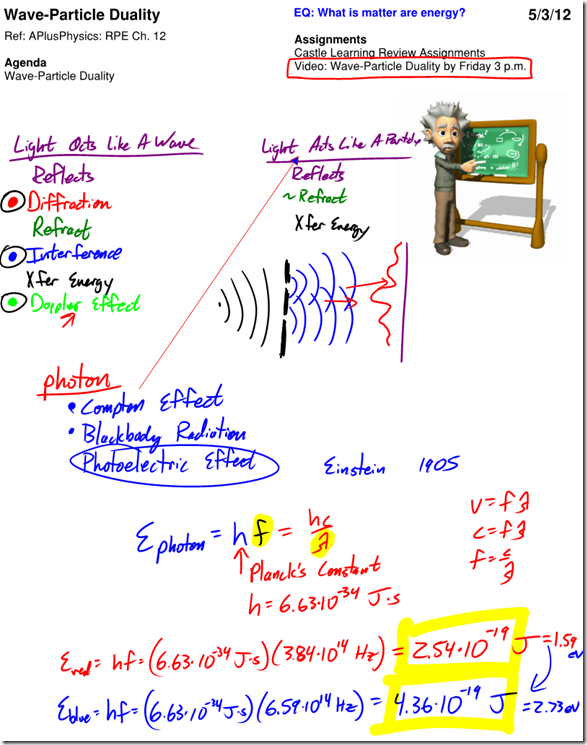
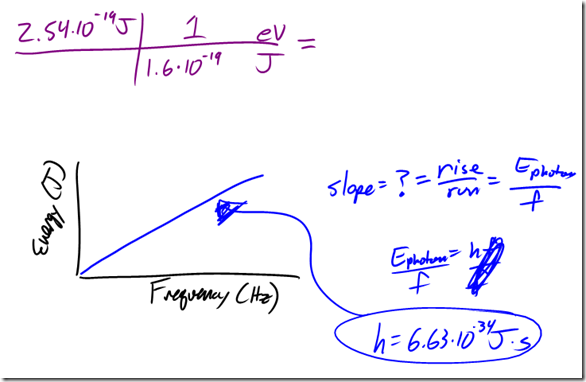
Wave-Particle Duality Lecture
Compton Effect and de Broglie Wavelength
Einstein continued to extend his theories around the interaction of photons and atomic particles, going so far as to hypothesize that photons could have momentum, also a particle property, even though they had no mass.
In 1922, American physicist Arthur Compton shot an X-ray photon at a graphite target to observe the collision between the photon and one of the graphite atom’s electrons. Compton observed that when the photon collided with an electron, a photoelectron was emitted, but the original X-ray was also scattered and emitted, but with a longer wavelength (indicating it had lost energy).
Further, the longer wavelength also indicated that the photon must have lost momentum. A detailed analysis showed that the energy and momentum lost by the X-ray was exactly equal to the energy and momentum gained by the photoelectron. Compton therefore concluded that not only do photons have momentum, they also obey the laws of conservation of energy and conservation of momentum!
In 1923, French physicist Louis De Broglie took Compton’s finding one step further. He stated that if EM waves can behave as moving particles, it would only make sense that a moving particle should exhibit wave properties. De Broglie’s hypothesis was confirmed by shooting electrons through a double slit, similar to Young’s Double Slit Experiment, and observing a diffraction pattern. The wavelength of a moving particle, now known as the De Broglie Wavelength, is given by: ![]() .
.
Blackbody Radiation
Blackbody Radiation*
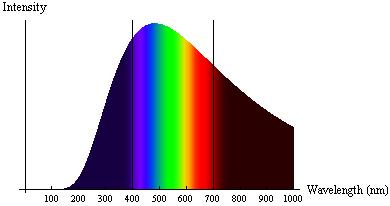
The radiation emitted from a very hot object (known as black-body radiation) didn’t align with physicists’ understanding of light as a wave. Specifically, very hot objects emitted radiation in a specific spectrum of frequencies and intensities, which varied with the temperature of the object. Hotter objects had higher intensities at lower wavelengths (toward the blue/UV end of the spectrum), and cooler objects emitted more intensity at higher wavelengths (toward the red/infrared end of the spectrum). Physicists expected that at very short wavelengths the energy radiated would become very large, in contrast to observed spectra. This problem was known as the ultraviolet catastrophe.
German physicist Max Planck solved this puzzle by proposing that atoms could only absorb or emit radiation in specific, non-continuous amounts, known as quanta. Energy, therefore, is quantized – it only exists in specific discrete amounts. For his work, Planck was awarded the Nobel Prize in Physics in 1918.
Regents Physics SBG Objective Tracking Sheets
10 Quick Tips to Maximize your Regents Physics Score
Although by no means an exhaustive list, these 10 quick tips may help you secure that extra point or two on your upcoming Regents Physics exam.
- Mass and inertia are the same thing.
- To find the resultant, line your vectors up tip-to-tail, and draw a line from the starting point of the first vector to the ending point of the last vector.
- Any object moving in a circular path is accelerating toward the center of the circle.
- Acceleration of an object is equal to the net force on the object divided by the object’s mass.
- The normal force always points at an angle of 90° from the surface.
- Opposite charges and magnetic poles attract, likes repel.
- Gravitational forces and electrostatic forces both follow an inverse square law relationship, where the strength of the force is related to one divided by the square of the distance between the charges/masses.
- The force of gravity on an object, commonly referred to as weight, is equal to mg, where g is the gravitational field strength (also referred to as the acceleration due to gravity).
- The mass-energy equivalence can be calculated using E=mc^2. If a mass is given in universal mass units, however, you can do a straight unit conversion using 1u = 931 MeV.
- Protons and neutrons fall into the category of baryons, which are hadrons. Smaller particles, such as electrons, fall into the category of leptons. Mesons are rare, weird particles you probably haven’t heard of.
Most importantly, use your reference table. When in doubt, write down the information you’re asked to find, what you’re given, and use your reference table to help you narrow down what you should be doing. In the free response part of the test, make sure to show your work in detail with a formula, substitution with units, and an answer with units.
Find these and many more tips for success at APlusPhysics.com.