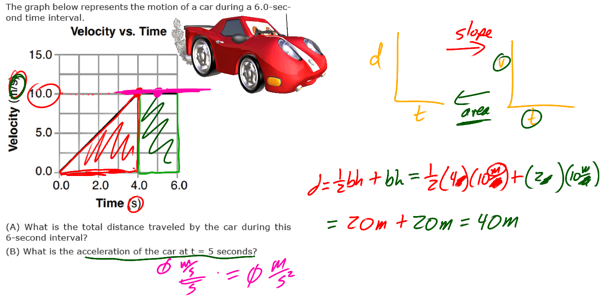
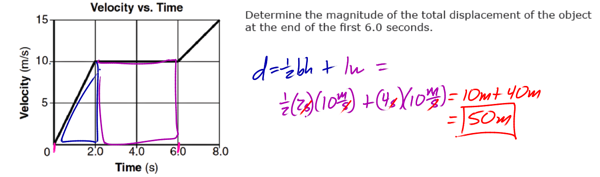






Modern Physics refers largely to advancements in physics from the 1900s to the present, extending the models of Newtonian (classical) mechanics and electricity and magnetism to the extremes of the very small, the very large, the very slow and the very fast. Modern Physics can encompass a tremendous variety of topics, which include:
Although electromagnetic waves exhibit many characteristics and properties of waves, they can also exhibit some characteristics and properties of particles. These “particles” are called photons. Light (and all EM radiation), therefore, has a dual nature. At times, light acts like a wave, and at other times it acts like a particle.
Characteristics of light that indicate light behaves like a wave include: Diffraction, Interference, Doppler Effect, and Young’s Double-Slit Experiment.
Characteristics of light that indicate light also acts as a particle include Blackbody Radiation, the Photoelectric Effect, and the Compton Effect.
“God does not play dice with the cosmos.” — Albert Einstein
“Einstein, don’t tell God what to do.” — Niels Bohr
Further evidence that light behaves like a particle was proposed by Albert Einstein in 1905. Scientists had observed that when EM radiation struck a piece of metal, electrons could be emitted (known as photoelectrons). What was troubling was that not all EM radiation created photoelectrons. Regardless of what intensity of light was incident upon the metal, the only variable that effected the creation of photoelectrons was the frequency of the light. If energy exists only in specifi c, discrete amounts, EM radiation exists in specifi c discrete amounts, and these smallest possible “pieces” of EM radiation are known as photons. A photon has zero mass and zero charge, and because it is a type of EM radiation, its velocity in a vacuum is equal to c (3×108 m/s). The energy of each photon of light is therefore quantized and is related to its frequency by the equation:
![]()
In this equation, the value of h, known as Planck’s Constant, is given as 6.63×10-34 J•s. Einstein proposed that the electrons in the metal object were held in an “energy well,” and had to absorb at least enough energy to pull the electron out of the energy well in order to emit a photoelectron. The electrons in the metal would not be released unless they absorbed a single photon with that minimum amount of energy, known as the work function (φ) of the metal. The frequency of this photon is known as the cutoff frequency of the metal. Any excess absorbed energy beyond that required to free the electron became kinetic energy for the photoelectron.
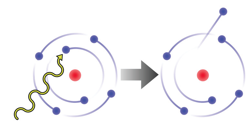
When a high-energy photon of light with energy greater than the energy holding an electron to its nucleus is absorbed by an atom, the electron is emitted as a photoelectron. The kinetic energy of the emitted photoelectron is exactly equal to the amount of energy holding the electron to the nucleus (the work function) subtracted from the energy of the absorbed photon.
![]()
This theory extended Planck’s work and inferred the particle-like behavior of photons of light. Photoelectrons would be ejected from the metal only if they absorbed a photon of light with frequency greater than or equal to a minimum threshold frequency, corresponding to the energy of a photon equal to the metal’s “electron well” energy for the most loosely held electrons. Regardless of the intensity of the incident EM radiation, only EM radiation at or above the threshold frequency could produce photoelectrons.
