Bright Lights and Physics, as one
Over the summer, I learned a lot about light, and I welcome the opportunity to share that with you. One thing I learned about was how different light sources stacked up against one another. Most people know that incandescent bulbs (tungsten-halogen, the "normal" kind) are less efficient that LED or fluorescent bulbs, but don't know why. I'm here to alleviate that knowledge gap.
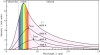
The reason incandescent bulbs are inefficient is because they produce a lot of heat - not just their own heat, but rather they radiate heat, in the form of infrared light waves. Eventually, all of the energy that goes into heating up the tungsten filament does get radiated out, but usually not at visible wavelengths. When an object is heated (through the addition of energy, AKA Q = mc * delta t), it begins to reemit that energy at a variety of wavelengths, until all that energy has been dispensed of. The distribution of this energy, as a function of wavelength, follows (mostly) a blackbody radiation distribution curve (see below), which shifts as a function of temperature. Most of the energy for a tungsten bulb is in the infrared region, meaning it is never actually seen. With hotter objects, however, more of the light is visible, and shifted towards lower wavelengths, which is why hot flames appear blue to our eyes.
A tungsten bulb is usually around 3300 degrees Kelvin, so a lot of energy is lost. However, in recent years, in attempts to save energy, other light producing methods have been pursued. One of these is the use of fluorescent bulbs, which rely on molecular excitation to produce light. As discussed in chemistry, electrons falling down an energy level emit photons of a very specific wavelength.
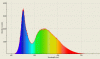
Above is an example spectral distribution curve. As you can see, almost all of the emitted light is in the visible region, making it much more efficient. However, because these contained dangerous chemicals oftentimes, the concept of the LED was also pursued as a potential light source. LEDs (a topic to be discussed by itself), "force" electrons to change energy levels as they flow through a circuit, through (usually) a junction between different types of doped silicon. The spectral distribution for these is, like the fluorescent, confined to a short wavelength band (usually blue). However, using a phosphor coating, which will absorb and reemit the light at different wavelengths, the light is made to look more "white" (see below).
That, in a nutshell, displays a variety of common lightings, and why some are preferred over others. I could go into more depth, but this gives a basic overview of how we light our world.




0 Comments
Recommended Comments
There are no comments to display.