Silicon
Semiconductors come in two types, elemental and compound. The elemental semiconductors come from column IV in the periodic table, and are silicon and germanium. Silicon is used more commonly than germanium due to its ability to withstand high temperatures and its ability to oxidize.
Semiconductors can also be made from compounds such as gallium arsenide, indium phosphide, and cadmium telluride. These compounds may be faster and more resistant to radiation, yet silicon is still far and away the most popular semiconductor for the production of integrated circuits. Silicon is cheaper than its competitors. Silicon is easier to process than its competitors. And silicon is the 2nd most abundant element on earth (behind oxygen).
Silicon has an atomic number of 14, and has four valence electrons. This indicates that silicon forms primarily covalent bonds, and when found in its pure crystalline form, each silicon atom shares an electron with its four nearest neighbor silicon atoms. The shared electrons are very tightly bound to the atoms, leaving very few electrons free to wander throughout the solid. This makes pure single crystal silicon a fairly good insulator.
If you replace one of the silicon atoms in the lattice with an impurity atom known as a dopant, you can modify the electrical properties of the structure. By replacing a silicon atom with an atom that has five valence electrons, from column five of the periodic table, such as phosphorus, arsenic, or antimony, four of the electrons would be required for covalent bonding, leaving an extra electron "free" to wander the lattice. These dopant atoms are known as donors, because they "donate" their extra electron to the lattice, effectively increasing the number of negative charge carriers in the solid, which increases the material's conductivity (and decreases its resistivity).
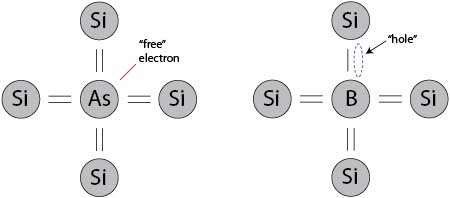
In similar fashion, if you were to replace one of the silicon atoms in the lattice with an atom that has three valence electrons, from column three of the period table, such as boron, indium, gallium, or aluminum, three of the electrons would be used up in covalent bonding, leaving a hole where a bond was previously. Electrons can jump in and fill that hole, leaving a hole elsewhere, in effect allowing the hole to move, therefore you have created a positive charge carrier, which also increases the material's conductivity and decreases its resistivity. These dopant atoms are known as acceptors, because they accept an electron from the lattice.
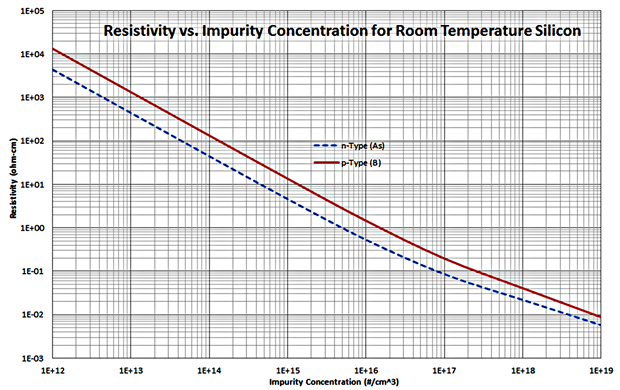
When silicon has been doped with more donors than acceptors, you have an excess of free electrons, or negative charges. This is called n-type silicon. When silicon has been doped with more acceptors than donors, providing an excess of free holes, or positive charges, it is called p-type silicon. Donor atoms are therefore also known as n-type dopants, and acceptor atoms are also known as p-type dopants. Note that in the case of both n-type and p-type dopants, the material as a whole remains neutral because the extra charge carriers are offset by the charge of the dopant nuclei.
Question: Magnetic-card door locks utilize many electronic components on one small piece of semiconductor material. This combination of components on a single chip is called
- a transistor
- an integrated circuit
- a printed circuit board
- a diode
Answer: (2) an integrated circuit
Question: Current in a semiconductor is caused by the movement of
- electrons only
- holes only
- isotopes
- both electrons and holes
Answer: (4) both electroncs and holes
Question: An impurity that is added to a semiconductor in order to provide holes is classified as a
- donor
- receptor
- acceptor
- bias
Answer: (3) acceptors accept an electron, leaving a hole.

